Understanding the Alzheimer's-Parkinson's link
UCI researchers find the neurodegenerative combo of Alzheimer’s and Parkinson’s diseases accelerates dementia.
On their own, Alzheimer’s disease and Parkinson’s disease cause the slow regression of mental and physical abilities. But when the proteins behind these neurodegenerative disorders join forces, a dramatic decline in cognition often results, researchers with UC Irvine’s Institute for Memory Impairments and Neurological Disorders have found.
Little has been known about how these disease-inducing proteins interact and influence mental processes, but a group headed by Frank LaFerla, Chancellor’s Professor of neurobiology & behavior and UCI MIND director, is beginning to change that.
Their work is generating hope for people with the Lewy body variant of Alzheimer’s, in which Parkinson’s symptoms are also present. Nearly half of all Alzheimer’s patients have this variant, and about 1.3 million of them in the U.S. suffer from Lewy body dementia.
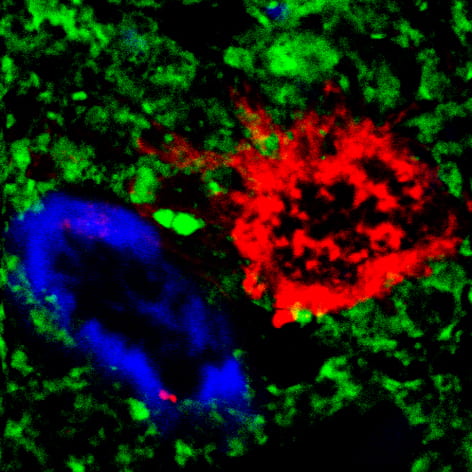
To learn more about this Alzheimer’s-Parkinson’s phenomenon, the LaFerla group created the first genetically modified, or “transgenic,” mouse model of this Lewy body variant of Alzheimer’s. As with affected patients, this model develops the beta-amyloid protein plaques and tau protein tangles typical of Alzheimer’s while also accumulating Parkinson’s-related alpha-synuclein proteins. These proteins aggregate into what are called Lewy bodies, named after Friederich H. Lewy, the scientist who discovered them.
“Mouse models such as this are opening a window to the complex biological activity that underpins neurodegenerative diseases like Alzheimer’s and Parkinson’s,” LaFerla says. “With enhanced research methods, we’re increasing our abilities to discover treatments.”
Using the new mouse model, he and UCI researchers Lani Clinton, Mathew Blurton-Jones and Kristoffer Myczek examined the interaction among these three proteins to determine how they influence cognition. The results of their study appear in the May 26 issue of The Journal of Neuroscience.
“We found the combination of these proteins in the same mouse accelerated the onset of the diseases,” says Blurton-Jones, an assistant researcher in neurobiology & behavior. “The proteins appeared to exacerbate ‘clumping up.’”
This aggregation, he says, led to a more rapid decline in the mice’s memory performance, similar to that in people with the Lewy body variant of Alzheimer’s.
“We’re hopeful that by better understanding and modeling the interactions among these proteins,” he says, “researchers can develop new treatments to target their activity.”
LaFerla and his UCI colleagues are on the forefront of utilizing genetically modified mice to study neurodegenerative diseases. In 2003, LaFerla created the first mouse model to accumulate beta-amyloid plaques and tau tangles, believed to be the pathological agents of Alzheimer’s disease.
“Everyone uses the mouse,” said Benjamin Wolozin, professor of pharmacology at Boston University’s Alzheimer’s Disease Center, in a 2006 profile of LaFerla in UCI Magazine. “His mouse is among the most important tools available to Alzheimer’s researchers.”
With the model, LaFerla has shown that beta-amyloid in particular may be the initiating factor in both kinds of Alzheimer’s – the “sporadic” type that seems to randomly attack the elderly and the early-onset “familial” version.
Last year, experiments by Blurton-Jones and LaFerla demonstrated that neural stem cells can partially reverse cognitive impairment in these genetically altered mice. The two subsequently received a $3.6-million California Institute for Regenerative Medicine grant toward the development of an Alzheimer’s therapy involving human neural stem cells.
“It’s absolutely critical to discover new ways to help people with Alzheimer’s, which is the leading cause of dementia among the elderly and affects 5.3 million people in the U.S., more than 500,000 of whom live in California,” LaFerla says. “We’re very excited about the advances spurred on by our development of transgenic mice, which we believe will lay the groundwork for Alzheimer’s treatments.”