UCI receives up to $5 million to advance bloodstream infection detection technology
A UC Irvine research team will receive up to $5 million over five years in federal support to further develop a bloodstream infection detection system that speeds up diagnosis times with unprecedented accuracy – allowing physicians to treat patients with potentially deadly ailments more promptly and effectively.
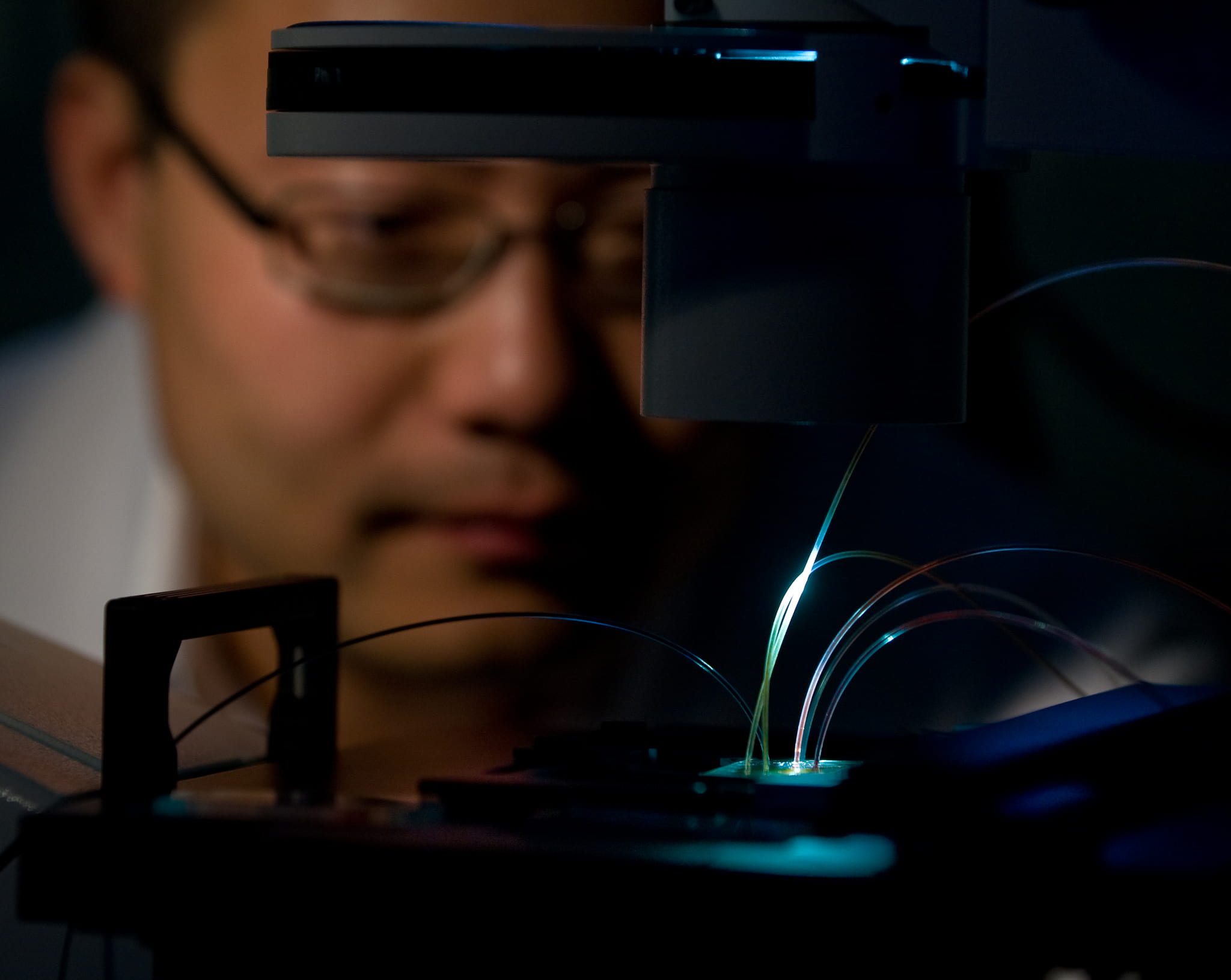
Irvine, Calif., April 30, 2015 — A UC Irvine research team will receive up to $5 million to further develop a bloodstream infection detection system that speeds up diagnosis times with unprecedented accuracy – allowing physicians to treat patients with potentially deadly ailments more promptly and effectively.
The five-year federal award is part of a National Institute of Allergy & Infectious Diseases program to fund nine institutions that will create tools to identify certain pathogens that frequently cause infections in healthcare settings – especially those that are resistant to most antimicrobials.
Advancing the development of quick and innovative diagnostic tests for identifying resistant bacteria is a key goal of President Barack Obama’s National Action Plan for Combating Antibiotic-Resistant Bacteria (pdf).
Led by Weian Zhao, assistant professor of pharmaceutical sciences, the UCI effort will employ his recently created Integrated Comprehensive Droplet Digital Detection system (IC 3D). In as little as 90 minutes, it can detect bacteria in milliliters of blood with single-cell sensitivity; no cell culture is needed.
“Rapid, precise diagnostics is vitally important to limit the spread of infections in the healthcare environment and to provide patients the prompt and exact treatments they need,” Zhao said. “In addition, this is an important part of meeting the president’s goal of personalized medicine. At the heart of this goal is giving the right patient the right drug at the right time. With rapid detection technology like IC 3D, this can be achieved.”
The IC 3D device will be used at UC Irvine Medical Center to identify in blood samples such antibiotic-resistant bacteria as extended spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, methicillin-resistant Staphylococci aureus and vancomycin-resistant Enterococci.
The clinical effort will be headed by Dr. Susan Huang, UCI professor of medicine and medical director of epidemiology and infection prevention, and Ellena Peterson, a UCI professor of pathology who oversees the clinical microbiology lab at UC Irvine Medical Center.
Bloodstream infections are a major cause of illness and death. In particular, infections associated with antimicrobial-resistant pathogens are a growing health problem worldwide. According to the Centers for Disease Control & Prevention, more than 2 million people a year in the U.S. get antibiotic-resistant blood infections, causing about 23,000 deaths.
“The mortality rate for blood infections is due, in part, to the inability to rapidly diagnose and treat patients in the early stages,” said Huang. A nationally recognized infectious diseases expert, she helps lead a CDC research program to develop and test innovative approaches to reducing infections in healthcare settings.
“This new technology will help advance early therapy,” she said. “It will have the potential to increase the survival of patients who are ill due to antibiotic-resistant pathogens.”
The IC 3D technology differs from other diagnostic techniques in that it converts blood samples directly into billions of very small droplets. Fluorescent DNA sensor solution infused into the droplets detects those with bacterial markers, lighting them up with an intense fluorescent signal.
Zhao noted that separating the samples into so many small drops minimizes the interference of other blood components, making it possible to directly identify target bacteria without the purification typically required in conventional assays.
A patent application has been filed by the University of California for the IC 3D technology, which has been optioned by Velox Biosystems, a startup company founded by Zhao to commercialize the product.
Other participants from UCI are Enrico Gratton, professor of biomedical engineering and director of the Laboratory for Fluorescence Dynamics; Michelle Digman, assistant professor of biomedical engineering; and Dong-Ku Kang, a pharmaceutical sciences project scientist.
The team will also work with industrial partners ISS Inc., Dolomite Microfluidics and BioVenture Services LLC to further develop and validate the IC 3D system and to modify the product with an eye toward future clinical approval.
About the University of California, Irvine: Currently celebrating its 50th anniversary, UCI is the youngest member of the prestigious Association of American Universities. The campus has produced three Nobel laureates and is known for its academic achievement, premier research, innovation and anteater mascot. Led by Chancellor Howard Gillman, UCI has more than 30,000 students and offers 192 degree programs. It’s located in one of the world’s safest and most economically vibrant communities and is Orange County’s second-largest employer, contributing $4.8 billion annually to the local economy. For more on UCI, visit www.uci.edu.
About UC Irvine Medical Center: Orange County’s only university hospital, UC Irvine Medical Center offers acute- and general-care services at its new, 482,000-square-foot UC Irvine Douglas Hospital and is home to the county’s only Level I trauma center, American College of Surgeons-verified regional burn center and National Cancer Institute-designated comprehensive cancer center. For 12 consecutive years, U.S. News & World Report has counted UC Irvine among “America’s Best Hospitals.”